The Complement System
보체계

What Is the Complement System?
Our bodies employ a variety of countermeasures against pathogens. Among the most important, but not well-known, is the complement system. It consists entirely of proteins, made mostly by the liver, which can alert other cells, aid phagocytosis by cells like macrophages and neutrophils, and can directly kill the invaders. Let’s explore how the complement system works, how it is initiated, and how it is terminated.
The Three Pathways
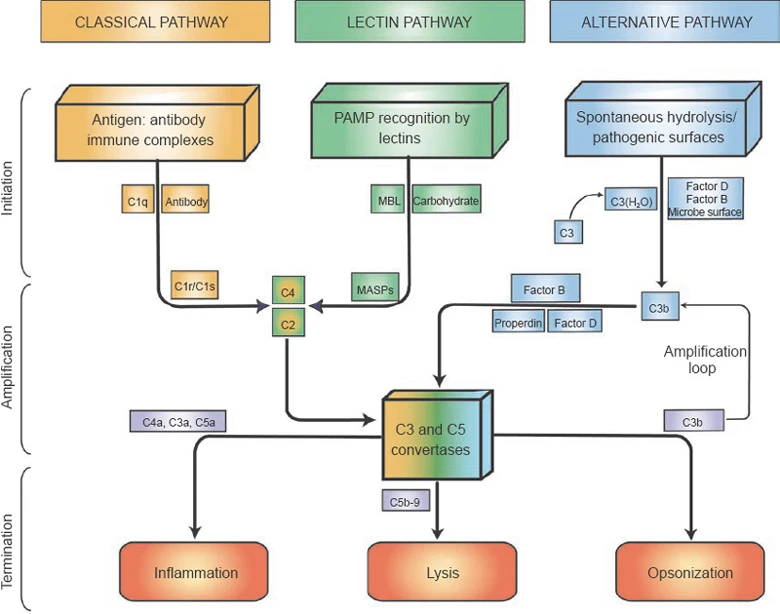
The complement system is a part of innate immunity. It is not antigen specific, unlike the components of the adaptive immunity, although it is influenced by antibodies. There are three pathways to initiate the complement system, the lectin pathway, the classical pathway, and the alternative pathway. One note before we start: all of them result in the formation of ‘C3 Convertase’, which is shared between the lectin pathway and the classical pathway and is different but has the same function in the alternative pathway. Also, we can infer from the naming of ‘C3 Convertase’ that it is an enzyme. Specifically, it has the purpose of converting a protein called 'C3'(Complement Protein 3) into C3a and C3b, C3b being the larger part.
All Three Pathways Result in the Cleavage of C3 into C3a and C3b, Which Triggers More Reactions
The Lectin Pathway
First is the lectin pathway. It is initiated when ‘MBL’ (‘Mannose Binding Lectin’, a protein complex made by around six trimers of mannose binding lectin monomers – Don’t get this wrong! There are MBL monomers which are simple rods with carbohydrate-binding domains. Three of these combine to form a thicker stalk, the MBL trimer. Finally, around six of these stalks combine to form an umbrella shaped MBL) binds to a mannose (a carbohydrate), which is only exposed on bacteria or fungi, and not on animals such as humans. The MBL complex then binds to another protein called MASP-2 (MBL Associated Serine Protease 2; ‘serine’ is an amino acid, and a ‘protease’ is a protein that cuts other proteins. Together, it means that a serine side chain in MASP-2 cuts another protein). The MASP-2 is activated by binding with the MBL and cleaves proteins ‘C4’ and ‘C2’, making ‘C4a’, ‘C4b’ and ‘C2a’, ‘C2b’ separately. C4b and C2a associate on the pathogen’s surface and creates ‘C 4b 2a’ (When complement system proteins are joined, their names are joined, too), which works as a ‘C3 convertase’!
- One note: some recent publishments use C2b as the larger fragment, and as the one that associates on the pathogen's surface, instead of C2a. This is a part of an effort to unite the larger, pathogen-binding part of cleaved proteins as 'b'. All other proteins have been historically named to follow this rule. Meanwhile, I used the historical naming convention of calling C2a as the larger, pathogen-binding fragment, following 'Janeway's Immunology, 10th Ed'.
The Classical Pathway
Second is the classical pathway, receiving naming from the fact that it was the first pathway discovered. The classical pathways directly receive help from the antibodies. Before we start, let's talk a little about antibody structure. An antibody looks like a scorpion with no barb and no legs. It has two binding parts and a conserved body part. The binding parts can have many, many configurations as we will discuss in depth in later posts. The binding parts are commonly called ‘Fab’, fragment antigen binding while the conserved part is called ‘Fc’, called fragment crystallizable. It’s called 'crystallizable' because after the antibody is broken down in a lab, that part crystallizes.
After an antibody attaches to a cell, an umbrella shaped complex, very similar to MBL, called ‘C1q’ attaches to the Fc part. Then, very similar to the lectin pathway, proteases are involved. C1q alters the shape of the integrated protease ‘C1r’ which is cleaved. C1r then cleaves another protease ‘C1s’. C1s cleaves C4 and C2, very similar to MASP-2, introduced earlier. C4b and C2a goes on to form the C3 Convertase, C4b2a. The larger C2a goes on to form the C3 convertase while the C2b floats away.
The Alternative Pathway
Third is the alternative pathway. The alternative pathway is dependent on a C3b. The C3b can result from the lectin pathway, classical pathway, or from an occasional split of C3 into C3a and C3b. This occasional split is called 'spontaneous hydrolysis', meaning it is split by water molecules on its own, not requiring ATP or additional enzymes. C3b attracts a protein ‘factor B’ and joins up to be ‘C3bB’. Finally, an enzyme called ‘factor D’ cleaves the B part of ‘C3bB’, making ‘C3bBb’. C3bBb works as a C3 convertase, further creating C3b and C3a. As we can see, the alternative pathway works to amplify the effects of a single C3b.
The Resulting Effects and the Terminal Reactions
We have seen three starting points of the complement system, all resulting in the C3 convertase, which has two types, C4b2a and C3bBb, and has the same function. We will now see its effects: pathogen-immune cell binding, signaling molecules, and the MAC. For these to occur, another set of reactions is needed. C3b can bind to both C3 convertases and result in ‘C4b2a3b’ and ‘C3bBb3b’. Both are called ‘C5 convertases’, which convert 'C5' into ‘C5a’ and ‘C5b’.
Signaling
Until this point, we have nearly always focused on the larger fragments: C4b, C3b, C5b. However, the smaller C4a, C3a, C5a work as signaling molecules. They give signals to immune cells to devour the nearby pathogen, and make more of them leave the bloodstream, by making them stick to the blood vessel walls. Specifically, the C3b makes the bacteria easy to bind with the macrophages and if the macrophage senses the C5a, it stimulates phagocytosis.
MAC
At last, we reach the final part of the complement system, the membrane attack complex (MAC). Starting from C5b, it binds to ‘C6’ and ‘C7’ and binds to the membrane of the pathogen. Another protein, C8 binds to the C5b, C6, C7 and is inserted into the membrane. Long ‘C9’ proteins are joined up with the C8 and make an irreversible hole in the pathogen, which can even kill if the pathogen’s cell walls are thin. Be aware that MAC is effective against some viruses and gram-negative bacteria which lacks a thick outer layer above the plasma membrane. It is known to be ineffective against pathogens with thick outer layers, such as gram-positive bacteria.
How Our Body Cells Are Not Affected
Our body uses many ways to stop the complement system from backfiring. For example, ‘factor H’ blocks the C3 convertase in the alternative pathway and ‘factor I’ with factor H cuts C3b and C4b making it harmless. Furthermore, it is notable that C3b has a short time to bind with the pathogen. C3b's binding region is quickly blocked by water molecules, making the alternative pathway localized on the pathogen, stopping damage to host cells.
Final Thoughts
The complement system is one example of how fascinating biology is. It is a coordinated effort by so many proteins to protect us. Similar cases exist throughout biology, such as the interaction between helper T cells and B cells, the recombination of genes, and finally us, who exist by the synced effort of 37 trillion cells.
Additional Materials & References
References
- Janeway’s Immunobiology, 10th Edition
- Wikipedia, ‘Complement system’ and related articles
- Tiny Bombs in your Blood - The Complement System, Kurzgesagt – In a Nutshell
Additional Graphics
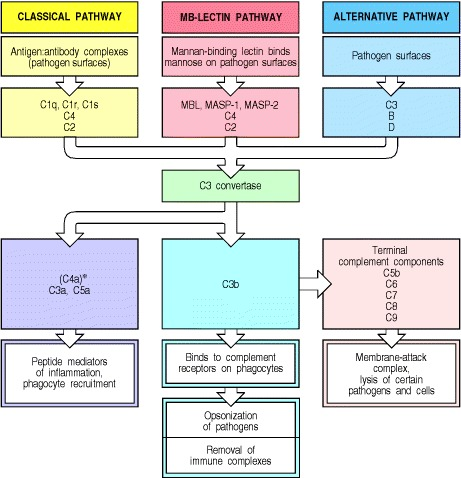
Source: Janeway's Immunobiology, 5th Edition, 2-5 Figure 2.8



Comments ()